Tigerfish User Tutorials
Overview
These tutorials for Tigerfish are intended to provide users with an overview of the tool’s features and functionality. Here, users will learn how to run Tigerfish using “Repeat Identification Mode”, “Probe Design Mode”, and Probe Analysis Mode using example cases and real data in the CHM13 v2.0 genome.
This tutorial guide was written in mind for those who are newer to running these scripts in a command line environment. These tutorials will help users ensure that they have installed Tigerfish properly on their own command line system.
So let’s get started! 🔬 🐯
Background
The following tutorial is used to cover Tigerfish functionality on a toy genome composed of the chr4 D4Z4 and chrX DXZ4 repeats which is derived from the latest version (v2.0) of the CHM13 human genome.
This reference file can be found within the project’s Github repo here.
Users will download the full CHM13 v2.0 reference file later in this tutorial, to work on real-world data.
For the test genome cases, there are three directories that exist which will be referenced in this tutorial.
First, we will walk through Repeat Identification Mode on a test genome and this directory is located here.
Next, we will move through the Probe Design Mode directory which can be found here.
Following this, we will work through Probe Analysis Mode using the same test genome which can be found here.
Lastly, we will move into the real-world examples in the main pipeline with CHM13 v2.0 using Probe Design Mode from the newly annotated chr9 HSAT repeat which is found here.
It’s important to note that the first three test directories are static meaning that the config.yml files have been organized to run Tigerfish for expected behavior in each directory. Therefore, users only need to execute the run_pipeline.sh script contained in each directory.
At UW Genome Sciences, users have access to the departmental Sun Grid Cluster Engine, which is used to deploy cluster compute jobs. Templates for how to run Tigerfish with such a file system can be found at the root directory of the Tigerfish GitHub here. This is intended to be a generalizable template that may be used to run Tigerfish jobs in independent directories to run in any command line environment.
To begin, we will describe some context for how Tigerfish is deployed by covering the config.yml file and appropriate run_pipeline.sh scripts found in each directory.
Config file
Tigerfish is a command line workflow that is implemented using Snakemake. This config.yml file summarizes parameters that users are able to modify.
However, we provide default parameters summarized in our paper for recommended use. These parameters for probe design are also described within the command line definitions page.
Expected Input and Outputs
Inputs
To run Tigerfish, users only need to provide the full sequence of a genome assembly in FASTA format and an accompanying chrom.sizes file that details the scaffolds present in the genome assembly and their lengths in bp.
Optionally, an addtional BED-formatted file that specifies genomic coordinates for interval(s) to perform probe design may be supplied.
All input files must be specified in the Tigerfish config.yml file for expected behavior.
Outputs
The Tigerfish pipeline generates core output files and supplementary output files.
Within the core output files, two key files summarize:
All candidte probes designed against target interval(s) if valid sequences exist in a tab separated text file.
The format of the candidate probe output file is described as follows:
Col |
Description |
|---|---|
1 |
Probe coordinates |
2 |
Interval coordinates |
3 |
Imaging target coordinates |
4 |
Probe Sequence |
5 |
Tm |
6 |
Aggregate probe k-mer sum in target interval |
7 |
Aggregate probe k-mer sum in target genome |
8 |
k-mer binding score (cols 6/ cols 7) |
9 |
Interval probe rank |
10 |
On-target probe binding score |
11 |
Off-target probe binding score |
12 |
On-target probe binding proportion |
13 |
Scaffold |
A summary file that describes the aggregate number of probes designed against each interval and their aggregate on-target binding scores in a tab separated text file.
Col |
Description |
|---|---|
1 |
Interval coordinates |
2 |
Total probes designed within interval |
3 |
Aggregate on-target binding for all probes within interval |
4 |
Aggregate off-target binding for all probes within interval |
Within the supplementary output files a set of auxiliary files that provide more detailed information about predicted binding profiles. To see test examples of whether Tigerfish was executed properly on sample files, each directory contains a supplementary shell script that may be run and will check the pipeline_output to the expected_output directories once the test directories have been executed.
Repeat Identification Mode on a Test Genome
Repeat Identification Mode is intended to be used when a user provides a given genome FASTA file and is perhaps unsure of where their target repeat regions of interest lie within the genomic sequence. Another valid use case for this option is if a user wants to perform genome-wide probe mining over all regions that Tigerfish deems as repetitive. In this case, the example genome FASTA contains a small subset of the DXZ4 and D4Z4 repeats.
Here’s a walkthrough of all the input files provided to get started with running Tigerfish in this example case:
Pipeline input
Genome FASTA reference file
chrom.sizes file
Bowtie2 indices (optional)
The test genome file described as example.fa:
The test genome chrom.sizes file described as test_chrom.sizes:
The Bowtie2 directories for this test genome reference which are found in the path data/bt2/ relative to the config.yml file:
Note: Bowtie2 directories against the queried genome are optional to provide. If you want this made de novo, you can specify this by toggling the relevant Bowtie2 flag in the config.yml file as shown below.
Pipeline output
All expected output files can be found within this directory.
Here, a collection of probes for both repeats found on each scaffold are provided in independent directories.
Pipeline executables
The config.yml file which has preset parameters that do not need to be modified for proper execution:
#path to genome fasta
fasta_file: "data/example.fa"
#path to file containing primary chromosome sizes
chrom_sizes_file: "data/test_chrom.sizes"
#if coordinates are provided for probe design, file goes here
bed_file: "data/dxz4_synthetic.bed"
#option for probe design that directs pipeline implementation
defined_coords: "FALSE"
repeat_discovery: "TRUE"
probe_cand_binding: "FALSE"
bowtie2_indices_given: "TRUE"
jf_hash_given: "FALSE"
jf_count_given: "FALSE"
chrom_idx_given: "FALSE"
chrom_fasta_given: "FALSE"
assembly: "chm13"
bowtie2_dir: "data/bt2/"
jf_hash_dir: ""
jf_count_dir: ""
chrom_idx_dir: ""
chrom_fasta_dir: ""
probe_binding_file: ""
#all chromosomes present in bed file or required for probe discovery are listed here
samples:
- "chr4"
- "chrX"
#parameters for repeat_ID step
window: 4000
threshold: 5
composition: 0.25
file_start: 0
#parameters for probe_design step
min_length: 36
max_length: 41
min_temp: 42
max_temp: 47
#parameters for kmer_filter script
mer_val: 18
c1_val: 1
c2_val: 5
#parameters used for probe_mer_filter script
enrich_score: 0.50
copy_num: 10
#parameters used in alignment_filter script
genome_windows: 5000000
thresh_window: 100000
binding_prop: 0.70
target_sum: 5000
off_bin_thresh: 100
mer_cutoff: 0.95
bt2_alignments: 500000
max_pdups_binding: 0.90
seed_length: 15
model_temp: 69.5
min_on_target: 25
max_probe_return: 40
align_thresh: 10
ref_flag: 0
Note: It’s extremely important to list all specific scaffolds of interest where repeat discovery will happen. In this case since we are interested in designing probes against this entire test genome, we list both chromosomes as their names are found within the genome FASTA file. Here, you can see that “chr4” and “chrX” are listed appropriately. If one wanted to perform repeat discovery on just one of these scaffolds, the others need not be listed.
The run_pipeline.sh script is what is used to execute the pipeline:

To check if the expected output files match to what is generated after you run the pipeline you can use the script run_check_repeatID.sh:
Let’s walkthrough
Begin by opening your command line terminal and making sure that conda is installed for your system as described in the Install section on our Getting Started page. Please proceed with installing Mamba as needed to assist with Snakemake installation.
Clone the Tigerfish repo into an empty directory and create the active environment as shown on the Installation page to activate the snakemake_env. Here, I already have this conda environment installed which is why I received the CondaValueError. But now we are ready to navigate to our test directory!
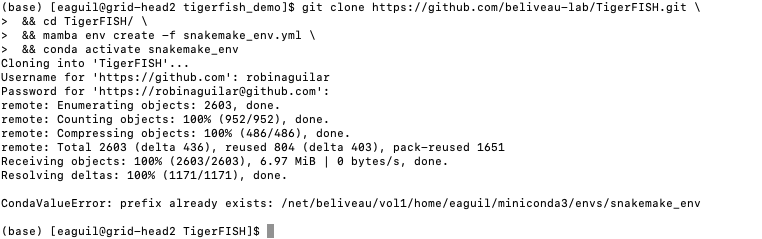
Navigate to the repeat discovery test directory which may be found here relative to the Tigerfish home directory:
cd example_run/repeat_discovery_test/
Within the repeat_discovery_test directory, you should be met with the following sub-directories and files once this command is executed:

Now all that is needed is to execute the run_pipeline.sh. This may be done by entering the following command:
. run_pipeline.sh
You will see that Tigerfish is solving and downloading relevant remote packages. This may take a few minutes to resolve before execution.
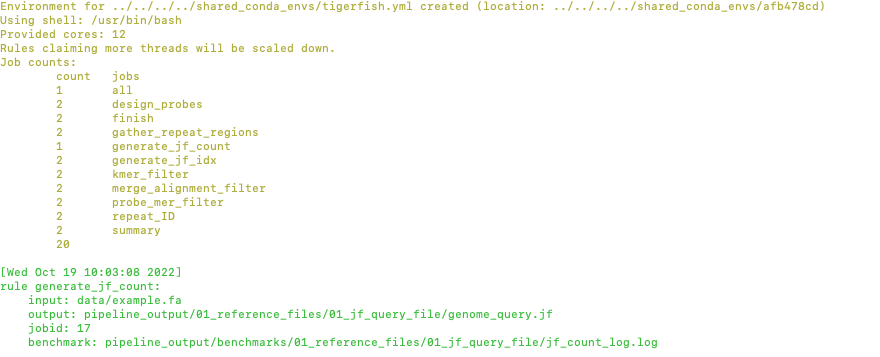
Now you can see that Tigerfish is successfully running! Output files will be populated in the pipeline_output/ directory. Which will be shown when you are greeted with the “DONE!” message.

If you want to compare if your files match what should be found in the expected output directories, you can run this check script like so:
. run_check_repeatID.sh
If everything is correct, this script will provide a message declaring: “Test run matches Tigerfish expected output!”. This is shown below:

Now you’re done! Congrats on running Repeat Discovery Mode! 🎉
If you happen to want to see a video of this happening as a real-time demo, you can watch this example here.
Now you’re ready to move into the Probe Design Mode tutorial!
Probe Design Mode on a Test Genome
Probe Design Mode is intended for users who know where their repeat target is and they are interested in probe design against a specific region or set of regions.
Pipeline Input
Genome FASTA reference file
chrom.sizes file
Bowtie2 indices (optional)
BED file of repeat region coordinates
To implement this run mode, users must also provide a BED file as the only additional input to what is described in the Repeat Identification Mode tutorial. Here, this BED file can be viewed in this directory. In this exercise, probes will only be designed against the selected DXZ4 repeat section.
Pipeline Output
All expected output files can be found within this directory.
Here, a collection of probes for the desired repeat region is provided in its own directory.
Pipeline executable
The config.yml file which has preset parameters that do not need to be modified for proper execution:
#path to genome fasta
fasta_file: "data/example.fa"
#path to file containing primary chromosome sizes
chrom_sizes_file: "data/test_chrom.sizes"
#if coordinates are provided for probe design, file goes here
bed_file: "data/dxz4_synthetic.bed"
#option for probe design that directs pipeline implementation
defined_coords: "TRUE"
repeat_discovery: "FALSE"
probe_cand_binding: "FALSE"
bowtie2_indices_given: "TRUE"
jf_hash_given: "FALSE"
jf_count_given: "FALSE"
chrom_idx_given: "FALSE"
chrom_fasta_given: "FALSE"
assembly: "chm13"
bowtie2_dir: "data/bt2/"
jf_hash_dir: ""
jf_count_dir: ""
chrom_idx_dir: ""
chrom_fasta_dir: ""
probe_cand_file: ""
#all chromosomes present in bed file or required for probe discovery are listed here
samples:
- "chrX"
#parameters for repeat_ID step
window: 4000
threshold: 5
composition: 0.25
file_start: 0
#parameters for probe_design step
min_length: 36
max_length: 41
min_temp: 42
max_temp: 47
#parameters for kmer_filter script
mer_val: 18
c1_val: 1
c2_val: 5
#parameters used for probe_mer_filter script
enrich_score: 0.50
copy_num: 10
#parameters used in alignment_filter script
genome_windows: 5000000
thresh_window: 100000
binding_prop: 0.70
target_sum: 5000
off_bin_thresh: 100
mer_cutoff: 0.95
bt2_alignments: 500000
max_pdups_binding: 0.90
seed_length: 15
model_temp: 69.5
min_on_target: 25
max_probe_return: 40
align_thresh: 10
ref_flag: 0
Note: It’s extremely important to list all specific scaffolds of interest where probe design will happen. In this case since we are interested in designing probes against chrX, we list this chromosome only as “chrX” as shown in the config.yml file.
Here, the provided organization of this directory where the Probe Design Mode tutorial takes place also contains a run_pipeline.sh script and run_check_defined_coords.sh. This structure mirrors the organization of the Repeat Discovery Mode test tutorial making this walkthrough fairly similar in behavior.
Let’s walkthrough
Return to the main home directory in the Tigerfish directory structure to enter the probe design mode test directory using the following command:
cd example_run/probe_design_test/
Enter the following command to execute the pipeline.
. run_pipeline.sh
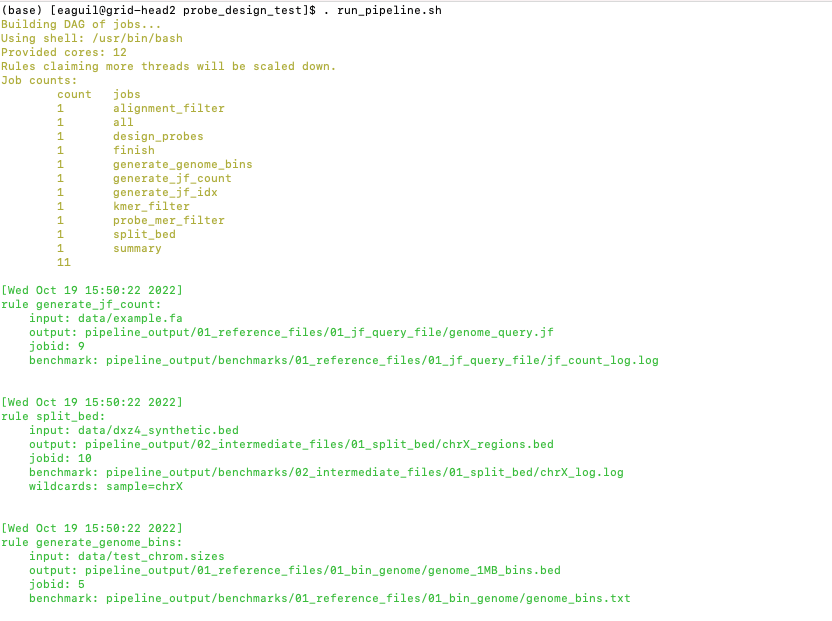
Now you will see that this pipeline has completed execution and you will receive a message declaring “DONE”!

To check if the pipeline output matches expected behavior, enter the following command to return the checked statement.
. run_check_defined_coords.sh
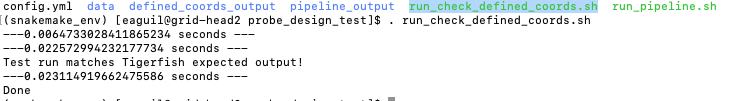
Great work! Congrats on running Probe Design Mode! 🎉 ✨
If you happen to want to see a video of this happening as a real-time demo, you can watch this example here.
Probe Analysis Mode on a Test Genome
Return to the main home directory in the Tigerfish directory structure to enter the probe design mode test directory using the following command:
cd example_run/probe_candidate_binding_test/
Enter the following command to execute the pipeline.
. run_pipeline.sh
Now you will see that this pipeline has completed execution and you will receive a message declaring “DONE”!
Nice! Congrats on running Probe Binding Mode! This means that all run modes of Tigerfish have passed in the test genome! 🎉 ✨
Comparing config.yml files between Repeat Discovery Mode and Probe Design Mode
It’s important to understand the distinct parameters that are being changed to toggle between Repeat Discovery Mode and Probe Design Mode. The key distinction are which parameters are being toggled to “TRUE” and “FALSE” for expected behavior. Let’s take a look at where these config.yml files differ:
Repeat Discovery Mode
#path to genome fasta
fasta_file: "data/example.fa"
#path to file containing primary chromosome sizes
chrom_sizes_file: "data/test_chrom.sizes"
#if coordinates are provided for probe design, file goes here
bed_file: "data/dxz4_synthetic.bed"
#option for probe design that directs pipeline implementation
defined_coords: "FALSE"
repeat_discovery: "TRUE"
probe_cand_binding: "FALSE"
bowtie2_indices_given: "TRUE"
jf_hash_given: "FALSE"
jf_count_given: "FALSE"
chrom_idx_given: "FALSE"
chrom_fasta_given: "FALSE"
assembly: "chm13"
bowtie2_dir: "data/bt2/"
jf_hash_dir: ""
jf_count_dir: ""
chrom_idx_dir: ""
chrom_fasta_dir: ""
probe_cand_file: ""
Probe Design Mode
#path to genome fasta
fasta_file: "data/example.fa"
#path to file containing primary chromosome sizes
chrom_sizes_file: "data/test_chrom.sizes"
#if coordinates are provided for probe design, file goes here
bed_file: "data/dxz4_synthetic.bed"
#option for probe design that directs pipeline implementation
defined_coords: "TRUE"
repeat_discovery: "FALSE"
probe_cand_binding: "FALSE"
bowtie2_indices_given: "TRUE"
jf_hash_given: "FALSE"
jf_count_given: "FALSE"
chrom_idx_given: "FALSE"
chrom_fasta_given: "FALSE"
assembly: "chm13"
bowtie2_dir: "data/bt2/"
jf_hash_dir: ""
jf_count_dir: ""
chrom_idx_dir: ""
chrom_fasta_dir: ""
probe_cand_file: ""
Here, the key difference in behavior can be controlled based on whether defined_coords = “TRUE” and repeat_discovery = “FALSE” to drive Probe Design Mode and vice versa for Repeat Discovery Mode. Be mindful that if one of these parameters is set to TRUE, the other must be set to FALSE or else the pipeline will be exited.
Probe Design Mode on chr9 HSAT in CHM13 v2.0
For this portion of the tutorial, we will be implementing probe design mode on real-world data using the CHM13 v2.0 genome to mine probes from the newly annotated chr9 human satellite (HSAT) repeat. Since we’ve already done a walkthrough of probe design mode on our test files, we will just begin with a walkthrough of how to begin running this process and what files need to me modified for this exercise.
Note: In the data directory you will see that the chrom.sizes file for this genome assembly and BED file with the chr9 HSAT coordinates are already provided.
For reference, we will be working in this directory shown here:
Let’s walkthrough
From the Tigerfish home directory, navigate to the following path:
cd example_run/probe_design_chm13/data
Next, we will download the CHM13 v2.0 genome into this directory. This genome build is found here. Under assembly releases, be sure to get the link for the file chm13v2.0.fa.gz. You can download the file using the following command. This may take a few moments.
wget https://s3-us-west-2.amazonaws.com/human-pangenomics/T2T/CHM13/assemblies/analysis_set/chm13v2.0.fa.gz
You can unzip this file by entering:
gunzip chm13v2.0.fa.gz
Now you need to update the path in the config.yml to execute the pipeline with the proper genome build path. So return out one directory to the config.yml file and include the path to the chm13 genome. This will look like the following:
#path to CHM13 genome fasta
fasta_file: ""
#path to file containing primary chromosome sizes
chrom_sizes_file: "data/chm13.chrom.sizes"
#if coordinates are provided for probe design, file goes here
bed_file: "data/chm13_chr9_hsat_array.bed"
#option for probe design that directs pipeline implementation
defined_coords: "TRUE"
repeat_discovery: "FALSE"
probe_cand_binding: "FALSE"
bowtie2_indices_given: "FALSE"
jf_hash_given: "FALSE"
jf_count_given: "FALSE"
chrom_idx_given: "FALSE"
chrom_fasta_given: "FALSE"
assembly: "chm13"
jf_hash_dir: ""
jf_count_dir: ""
chrom_idx_dir: ""
chrom_fasta_dir: ""
bowtie2_dir: ""
probe_cand_file: ""
#all chromosomes present in bed file or required for probe discovery are listed here
samples:
- "chr9"
#parameters for repeat_ID step
window: 4000
threshold: 5
composition: 0.25
file_start: 0
#parameters for probe_design step
min_length: 25
max_length: 50
min_temp: 42
max_temp: 52
#parameters for kmer_filter script
mer_val: 18
c1_val: 1
c2_val: 5
#parameters used for probe_mer_filter script
enrich_score: 0.70
copy_num: 40
#parameters used in alignment_filter script
genome_windows: 5000000
thresh_window: 100000
binding_prop: 0.70
off_bin_thresh: 100
target_sum: 20000
mer_cutoff: 0.95
bt2_alignments: 500000
max_pdups_binding: 0.90
seed_length: 15
model_temp: 69.5
min_on_target: 25
max_probe_return: 20
align_thresh: 10
ref_flag: 0
Now you are ready to run this process! For Sun Grid users, it is recommended that you submit this as a cluster job. Templates of how to do this to your shell script to make it executable for cluster job submissions in provided in the Tigerfish home directory.
Once completed, you can check this job process based on expected output. Here only the probe file was kept from the full expected output to minimize memory use. To check if the output matches what is in the expected output directory, simply run the following command:
. run_check_defined_coords.sh
Congrats! You’ve designed a real probe that will work to visualize the chr9 HSAT array uniquely!
Final thoughts
We hope this tutorial provides a comprehensive overview of what is needed to get users started with Tigerfish. Happy FISHing for new repeats!